|
Current Finding
From Rice Husk to Heterogeneous Catalyst
Rice husk
is a waste product of the agriculture activity in most countries in Asia
and in particular Malaysia. Rice husk has posed a major problem of disposal
to the rice milling industry in Malaysia and elsewhere in the world. Efforts
have been made in the past 20 years to use rice husk and its ash in various
ways [1]. Moreover, efforts have been made in recent times to extract
useful products from rice husk. It has been shown by many researchers
that rice husk contains 15 – 20% silica (SiO2), which contributes
to its hard and abrasive protective casing covering the rice grain. The
silica in rice husk can be obtained by removing the organic components
through control burning in muffle furnace in the laboratory. Our recent
studies have, however, yielded a more environment friendly technique,
whereby the silica from rice husk is obtained by solvent extraction (i.e.
without the need for burning) [2]. This chemical procedure has been developed
in our laboratory and we have filed a patent for the process. The silica
obtained was found to have a high specific surface area of 300 –
400 m2g-1. The purity was determined by x-ray fluorescence and it was
shown to be 99.99% pure silica. In contrast, the pyrolysis process produces
93-95% silica. This can be further purified to > 99% by treating the
silica with mineral acids like HCl, H2SO4 and HNO3. The silica, from pyrolysed
rice husk-ash (RHA) has been shown to adsorb fatty acids from palm oil
[3]. A model study showed the adsorption of fatty acids could be described
by the Langmuir isotherm [4].
Currently, we are studying the rice husk ash as
a possible heterogeneous catalyst. Several interesting results have emerged
form our studies. The RHA has been modified with selected transition metals
by co-precipitating the sodium silicate and the metal salt in nitric acid
so that the metal can be incorporated into the silica matrix. Tests by
SEM and EDX on the solids obtained show that the transition metals are
indeed incorporated chemically into the silica matrix. The resulting solid
was sometimes amorphous and at other times crystalline depending on “the
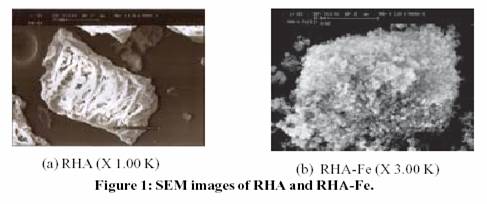
speed of titration (neutralization)”. Fig. 1 shows the SEM micrographs
of RHA and the iron-incorporated catalyst RHA-Fe. Initial experiments
conducted show promise for these new materials as heterogeneous catalyst.
The Fe loaded RHA-Fe was used in the Friedel-Craft benzylation of toluene
with benzyl chloride [5].
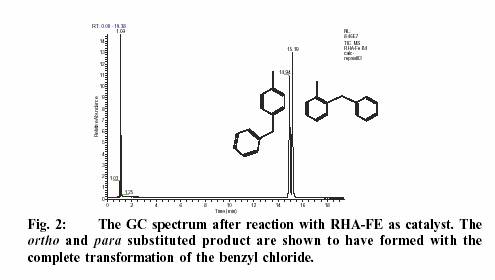
Fig. 2 shows the GC
spectrum of the reaction products. The peak at 1.09 is toluene, which
was present in excess. Benzyl chloride appeared at 2.33, which has been
completely converted to products. The ortho and para- substituted products
were successfully separated in the GR chromatogram. The mono substituted
product accounted for 93 % while the di-substituted product yield was
4 %. Recently 4-(methylamino)benzoic acid was incorporated together with
Fe [6] to form RH-Fe(amine). This catalyst was shown to reduce the di-substituted
products to ca. than 1%.
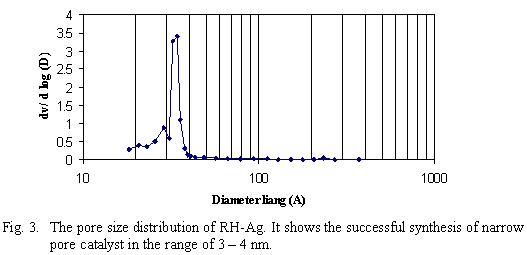
In a related study,
the incorporation of silver into RH resulted in RH-Ag. This resulted in
the formation of a narrow pore catalyst as shown in Fig. 3.
This catalyst was successfully used in the oxidation
of benzyl alcohol to benzaldehyde and dibenzyl ether.
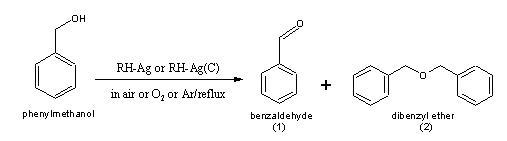
It was shown that under different condition of
the experiment, product (1 or (2) may predominate [7].
The chromium incorporated RH-Cr and RH-Cr(amine) was found to give
ca. 100 % conversion in the oxidation of cyclohexane into cyclohexanol
and cyclohexanone as shown bellow:

This is an important discovery
as cyclohexane conversions were reported to be never more than 50 % in
the literature. That too in many cases, the reaction were in the gas phase
at elevated temperatures and pressure. However, in our case the reaction
was conducted simply in a round bottom flask and refluxed at the boiling
point of the solvent, acetonitrile. The product distribution achieved
in the above reaction was ca. 50 % each. In our ongoing research, we are
looking into the selectivity and the mechanism of some of these catalyst.
References:
1.
Farook Adam, Ismail Ab. Rahman and M. I. Saleh, (1989). “Production
and characterization of rice husk ash as a source of pure silica”.
Seramik Nusantra: Proceedings of the first National Seminar on Ceramic
Technology, pp. 261-273.
2.
Farook Adam and Fua Hock Kiong, Unpublished results, (2003).
3.
Farook Adam, MSc Thesis, Universiti Sains Malaysia , (1992).
4.
F. Adam and S. Ravendran, J. Am. Oil Chem. Soc., 77 (2000)
437-440.
5.
Farook Adam, Kalaivani Kandasamy and Saraswathy Balakrishnan, “Iron
incorporated heterogeneous catalyst from rice husk ash”. J. colloid
and Interface Sci., 304 (2006) 137 – 143.
6. Farook Adam and Jeyeshelly Andas, “Amino benzoic acid modified
silica - an improved catalyst for the mono substituted product in the
benzylation of toluene with benzyl chloride”. J. Colloid and Interface
Sci. Accepted for publication (2007).
7. Farook Adam, Adil Elhag Ahmed and Sia Lih Min, “Silver
modified porous silica from rice husk and its catalytic potential”.
Journal of Porous Material. Accepted for publication (2007).
Consultancy/Public Service
The School of Chemical Sciences encourage cooperation between
its researchers and the industrial sector and other private agencies.
Consultancy work can be undertaken in all areas of chemical analysis (e.g.
AA, FT-IR, FT-NMR, elemental analysis, GC/MS, TGA etc.), product development,
synthesis and characterization of new and novel compounds which are of
benefit especially to the chemical industry and the general public can
be undertaken. Interested parties and prospective postgraduate students
are welcome to contact me directly for a friendly discussion.
My Research Group

Postgraduate Program in heterogeneous catalysis
(M.Sc./Ph.D.)
Currently we are actively doing research in the synthesis of organosilicon
esters using triphenyl silanol as the base alcohol. This is a very challenging
area of synthesis as no reports of silyl esters containing the triphenylsilanol
derivative have been reported. Students are also pursuing their postgraduate
studies in the synthesis of heterogeneous catalyst from rice husk and
its use in organic and inorganic fine chemical synthesis.
Interested candidates/organizations please contact me at:
School of Chemical Sciences
Universiti Sains Malaysia
11800 Minden
Pulau Pinang
Malaysia
Tel. (O): 04-6533888 ext. 3567
Fax (O): 04-6574854
E-mail: farook@usm.my or afarook@streamyx.com
|

