Interesting
Topics
SYNTHESIS, CHARACTERIZATION AND
UTILIZATION OF
CYCLOTRIPHOSPHAZENES AND NATURAL RUBBER DERIVATIVES
by
MAS ROSEMAL
HAKIM BIN MAS HARIS
Preamble
The reduction of land surface area for the cultivation of rubber trees demands both the industrial and academic sectors in Malaysia to diversify the utilization of natural rubber and its derivatives beyond their traditional usage. In view of this and the availability of funding, particularly in the form of Intensification of Research in Priority Areas (IRPA) grants for enhancing the national socio-economic position, I have focused my research effort on the development of new methods for imparting desired physical and chemical properties onto rubber and its derivatives. Highlights of three topics (sub-areas) of interest shall be presented to reflect our research contributions over the past ten years.
(I). Fire-Retardant Rubber Derivatives: Synthesis And Characterization Of Cyclotriphosphazene-Modified Natural
Rubber Derivatives
Cyclotriphosphazenes are compounds that contain
alternating phosphorus and nitrogen atoms, in a six-membered ring, with two
substituents on each phosphorus (Gleria and De Jaeger, 2001). They are used as monomers or precursors
for the development of high performance linear, cyclolinear and cyclomatrix
inorganic-organic polymers (Levchik et al., 1998). Furthermore, it is known that incorporation of cyclotriphosphazenes
as pendant groups to the backbone of organic polymer affords polymeric
materials, a class of organic-inorganic hybrid polymers, with good fire
retardant properties (Allen et. al., 1988; Inoue et. al., 1989;
Selvaraja and Chandrasekhar, 1997).
Mas and his students pioneered the technique of incorporating cyclotriphosphazenes (chemically bonded) onto the backbone of natural
rubber. Brief description of two
distinctive synthetic approaches is presented below.
In
the first approach (Loo, 1993) ,
1,3,3,5,5-(trifluoroethoxy)-1-oxocyclotriphosphazene moiety [N3P3(OCH2CF3)5O–]
is attached to the backbone of natural rubber by reacting [N3P3(OCH2CF3)5O-Na+]
(Lanoux and Mas, 1986) with 50 mole percent epoxidized natural rubber
(ENR-50) in boiling 1,4-dioxane for 72 hours. The nucleophilic reaction is depicted in Scheme 1. Addition of mildly acidic aqueous media to
the 1,4-dioxane solution afforded the cyclotriphosphazene-incorporated
natural rubber derivative, [P1].
Structure of [P1] was elucidated by means of elemental (C, H and N)
analysis, FT-IR spectroscopy and FT-NMR (1H, 13C and 31P)
spectroscopy. Quantitative 1H
NMR spectral analysis revealed that the relative number of olefinic moiety
remained practically unchanged.
Therefore, the nucleophile reacted exclusively with the epoxy moiety
of ENR-50.
In
the second approach (Zainab, 1995), ENR-50 was first reacted with meta-aminophenol
[m-H2N(C6H4OH)] in the presence of
catalytic amount of AlCl3.6H2O in 1,4-dioxane producing
a rubber derivative with reactive aromatic hydroxyl groups, [P2]. This intermediate product could be
isolated and reacted with (NPCl2)3 to give [P3]. The two-step reaction is depicted in Scheme
2. The second reaction could also be
performed in-situ by adding a 1,4-dioxane solution of (NPCl2)3
into the reaction flask together with an excess amount of triethylamine (Et3N:)
as HCl acceptor. The structures of
[P2] and [P3] were elucidated by a combination of analytical methods such as
elemental (C, H and N) analysis, FT-IR spectroscopy, 1D & 2D 1H
NMR spectroscopy, 13C and 31P NMR spectroscopy.

Scheme 1 Incorporation of cyclotriphosphazene units
onto the backbone of natural rubber derivative via
the reaction of [N3P3(OCH2CF3)5O-Na+]
with ENR-50

Scheme 2 Incorporation of
cyclotriphosphazene units onto the backbone of natural rubber derivative via
the reaction of ENR-50 with m-H2N(C6H4OH)
and with (NPCl2)3
Both
[P1] and [P3] exhibited markedly enhanced fire retardant properties. For instance, ENR-50, when ignited, burned
with dripping to completion, whereas the cyclotriphosphazene-modified rubber
derivatives, even after been soaked with a flammable solvent such as
kerosene, resisted burning under normal atmospheric conditions. Furthermore, the polymeric materials
exhibited self-extinguishing behavior.
A drawback of the above
two approaches is that the number of cyclotriphosphazene units that could be
incorporated is limited by the number of epoxy groups initially presence in
the organic polymer. Furthermore,
from structural point of view, both approaches resulted in the destruction
(even though only partially) of the original cis configuration of the
rubber derivative due to cleavage of the epoxy groups.
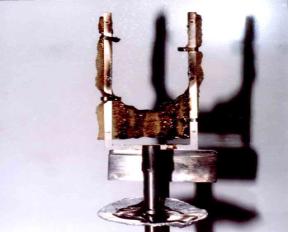
Recently, we have developed a new approach of imparting good thermal and fire retardant properties of phosphazene-based material onto natural rubber and its derivatives. This third approach involves the preparation of appropriate organic-inorganic hybrid networks to trap the polymer of interest (Salah, 2003; Lim 2003). Detail of this later approach is not described here. Suffice to say that it is possible to effectively trap epoxidized natural rubber in the phosphazene-based material networks thereby imparting desired thermal and fire retardant properties to the natural rubber derivative and yet keeping its original structure intact. For example, the burning behavior of neat ENR-25 and that of [P5/ENR25]s (a product resulted from the entrapment of ENR-25 in cyclomatrix poly(1,3-dioxybenzene)cyclotriphosphosphazene) is shown in Figure 1 and Figure 2, respectively.

(a) (b)
Figure
1 Burning
behavior of neat ENR-25: (a) early stage, burns with dripping (LOI = 18.2),
and (b) advanced stage, total lost of solid texture.


(a)
(b)
Figure
2. Burning
behavior of [P5/ENR25]s : (a) early stage, burns with difficulty
(LOI = 29.5), and
(b)
advanced stage, solid texture preserved.
(II) Preparation Of Water-Soluble Rubber Derivatives: Meeting The Challenge For Novel Utilizations Of Rubber In Aqueous Media
A novel method for the preparation of rubber derivatives that can be readily dissolved in water forming clear and stable solution (Figure 3) has been successfully developed (http://www.usm.my/r&d/frontiers/f1/2.html).


(a) (b)
Figure 3. (a) Rubber Derivative In
Emulsion (Latex) and Clear Aqueous Solution Forms
(b)Visual comparison: transmission of light through the rubber derivative solution vs latex forms
Some of the usages (advantages) of the products of this invention are outlined below:
ü Products of this invention can be used for cleaning water (containing very fine, unfilterable particulates such as smoke residue, carbon or wood dust, or finely dispersed liquid such as oil droplets or undesired pigmentation or pungent odors) in a very simple and cost effective manner.
ü Products of this invention can be used to extract or reclaim useful metal ions such copper, nickel, iron, tin, platinum, gold, chromium, mercury and lead ions from water.
ü Products of this invention can be used in conjunction with another ion removing material. For example, by adding to the aqueous media an ion removing material such as activated charcoal or an ion exchange resin or a zeolite or chitin or chitosan to trap the ions of interest and then adding the rubber solution followed by a simple flocculating and precipitating process. It is important to note here that minute particles of the ions removing material that would otherwise be difficult to remove after a conventional treatment can now, that is after the addition of the rubber solution, be easily removed.
ü Products of this invention can be used as slow-release binding matrices for fertilizers and pesticides. Fertilizers and pesticides in this form have the advantage that they are not washed so readily in a heavy downpour.
Dynamic chemical structures of the water-soluble derivatives were established with the aid of high-resolution Nuclear Magnetic Resonance (NMR) techniques. Samples were dissolved in deuterated water (D2O). Spectra at different temperatures between 5 oC and 95 oC were recorded (depicted in Figure 4 and Figure 5). At low temperature (<30 oC), only very broad or no resonance is observed in 1H NMR spectra while in 13C NMR spectra only resonances due to the methyl carbons (at 22.4 ppm and 23.4 ppm), the secondary oxirane carbon (at 60.5 ppm) and the alkenic carbons (at 124.8 ppm and 134.7 ppm) are observed. At high temperature (for example at 95 oC), resonances due to all type of protons (see Figure 4) and carbons (see Figure 5) present are readily discernible. Therefore, strong dynamic interaction between the carbon chains (polymer backbone) and water molecules (resulting in the formation of a clear and stable solution) is confirmed. For comparison, both the 1H and 13C NMR spectra of the unmodified ENR-50 dissolved in deuterated chloroform (CDCl3) were also obtained.

Figure 4. Variable temperature 1H
NMR spectra of a water-soluble product (modified ENR-50)

Figure 5. Variable temperature 13C
NMR spectra of a water-soluble product (modified ENR-50)
(III) Synthesis And Characterization Of New
Inorganic-Organic
Hybrid Materials With Good Fire Retardant and Adhesive Properties
A method for the preparation of a non-flammable, odorless adhesive that forms a transparent layer and provides very strong bond especially between glass-to-glass surfaces has been developed (I-TEX 2002 award). The product can be produced in solid or white powder form (Figure 6). Its adhesive property can be affected by melting it with a heat gun or a cigarette lighter (Figure 7) and compressing the resulting liquid with another piece of glass (Figure 8). Clear and strong adhesion of the two pieces of glasses will occur upon cooling (Figure 9).



Figure 6 Figure
7 Figure 8 Figure 9
Unlike
cellulose- and epoxy-based adhesives, this hybrid material is a reversible adhesive. Bonded surfaces can be unglued
and reglued with the same material repeatedly (the material can be readily
recovered and reused).
Information
on the thermal stability and adhesive properties (nature of bondings involved)
of this class of inorganic-organic hybrid material is still lacking. Work is progress to obtain a comprehensive
data by means of various techniques such as by FT-NMR, FT-IR, TGA, DSC, GPC,
SEM, EDX and X-Ray.
Conclusion
We have demonstrated that the thermal and fire retardant properties of natural rubber derivatives can be significantly improved by incorporating cyclotriphosphazenes as pendant units and by entrapping in appropriate phosphazene-based material networks. We have also demonstrated that it is possible to prepare water-soluble rubber derivatives. A method for the preparation of a non-flammable, odorless and reusable adhesive has also been developed.
“What has
motivated me to persistently stick to the product-oriented research all these
years is that I believe we can make ‘small’ R&D breakthroughs in
USM. More importantly is the desire
to pass on the knowledge to my students whom may bring this culture
(scientist cum inventor) to a greater height in the future”.
Acknowledgement
We are grateful to the Ministry of Sciences and Technology, Malaysia for providing the fund (IRPA grants) and to Universiti Sains Malaysia for providing the space and facilities to carry out the research projects.
References
Allen, C.W., Shaw, J.C. and Brown, D.E. (1988). Organophosphazenes. 22. Copolymerization of (Methylethenylphenyl)pentafluorocyclotriphosphazene with Styrene and Methyl Methacrylate. Macromolecules, 21(9), 2653-2657.
Gleria, M. and
Jaeger, R.D. (2001). Aspects of Phosphazene Research. Journal of Inorganic
and Organometallic Polymers, 11(1), 1-45.
Inoue, K., Nakamura, H., Ariyoshi, S.,
Takagi, M. and Tanigaki, T. (1989b). Heat Resistance Polymers Prepared from
[(4′-(2-Vinyl)-4-Biphenylyl)Oxy] Pentachlorocyclotriphosphazene. Macromolecules,
22(12), 4466-4469.
Lanoux, S. and Mas, R.H. (1986). Reactions
of the Hydrolyzed Phosphazene [N3P3(OCH2CF3)5
O-Na+]. Phosphorus and Sulfur, 26,
139-142.
Levchik, S.V., Camino, G., Luda, M.P.,
Costa, L., Lindsay, A. and Stevenson, D. (1998). Thermal Decomposition of
Cyclotriphosphazenes. I. Alkyl-Aminoaryl Ethers. Journal of Applied
Polymer Science, 67. 461-472.
Lim Eng Khoon, Modification Of Liquid Epoxidized Natural Rubber With Poly(1,5- dioxynapthalene)cyclotriphosphazenes, Master thesis, Universiti Sains Malaysia.
Loo, S.C. (1993). Synthesis and Characterization of polymer Derivatives from 50%-Epoxidized Natural Rubber and Sodium 1,3,3,5,5-pentakis(trifluoroethoxy)-1-oxocyclotriphosphazenate. Master thesis, Universiti Sains Malaysia.
Salah Mahdi M. Al-Shukri, Synthesis And Characterization Of Poly(1,3-dioxybenzene)-cyclotriphosphazenes And Their Utilization In The Enhancement Of The Thermal And Fire Retardant Properties Of 25-Mole Percent Epoxidized Natural Rubber PhD. Thesis, Universiti Sains Malaysia
Selvaraja, I.I. and
Chandrasekhar, V. (1997). Copolymerization of 2-(4-vinyl-4-biphenylyloxy)pentachlorocyclotriphosphazene
with Acrylate and Methacrylate Monomers. Polymer, 38(14). 3617-3623.
Zainab B.N. (1995).
The Incorporation of Pentachlorocyclotriphosphazene Into the Natural
Rubber Network Via Meta-Aminophenol. Master Thesis, Universiti Sains
Malaysia.